Chemical Oceanography
Marine chemists strive to understand the mechanisms and rates by which chemicals move through the marine environment. Many chemicals are there entirely naturally (e.g. salts from rivers and hydrothermal discharges at mid-ocean ridge crests), whereas others derive from human activities (e.g. lead, mercury, and persistent organic pollutants).
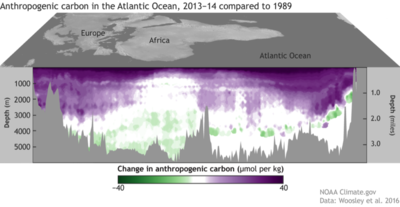
 The chemical oceanography group of Prof. Ed Boyle focuses on natural and anthropogenic metals in the ocean. For example, most of the lead in the ocean today comes from human activities (high temperature industrial processes and leaded gasoline utilization). Because the patterns of lead emissions are changing on decadal time scales (e.g. leaded gasoline utilization), the lead concentrations in the ocean water column are evolving in time (e.g. Pb in the upper water column near Bermuda). By using the historical record preserved in annually-banded corals, we are able to evaluate changes in oceanic surface water Pb over the anthropogenic era.
The chemical oceanography group of Prof. Ed Boyle focuses on natural and anthropogenic metals in the ocean. For example, most of the lead in the ocean today comes from human activities (high temperature industrial processes and leaded gasoline utilization). Because the patterns of lead emissions are changing on decadal time scales (e.g. leaded gasoline utilization), the lead concentrations in the ocean water column are evolving in time (e.g. Pb in the upper water column near Bermuda). By using the historical record preserved in annually-banded corals, we are able to evaluate changes in oceanic surface water Pb over the anthropogenic era.
In PAOC we also study the oceanic chemistry of iron, a biologically essential element that is in short supply in the ocean because of its low solubility. There is very little good data for iron in the ocean because of the enormous potential for sample contamination by rusty ships!